Copper Electron Configuration Anomaly . Cr = [ar] 4s1 3d5. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Learning outcome 2.3 c) justify the anomalous electronic. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 312 views 2 years ago 2.3 electronic configuration. Some of the elements have electron configurations that differ slightly from what our general. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. the actual electron configurations are: Cu = [ar] 4s1 3d10. cu has an anomalous electron configuration.
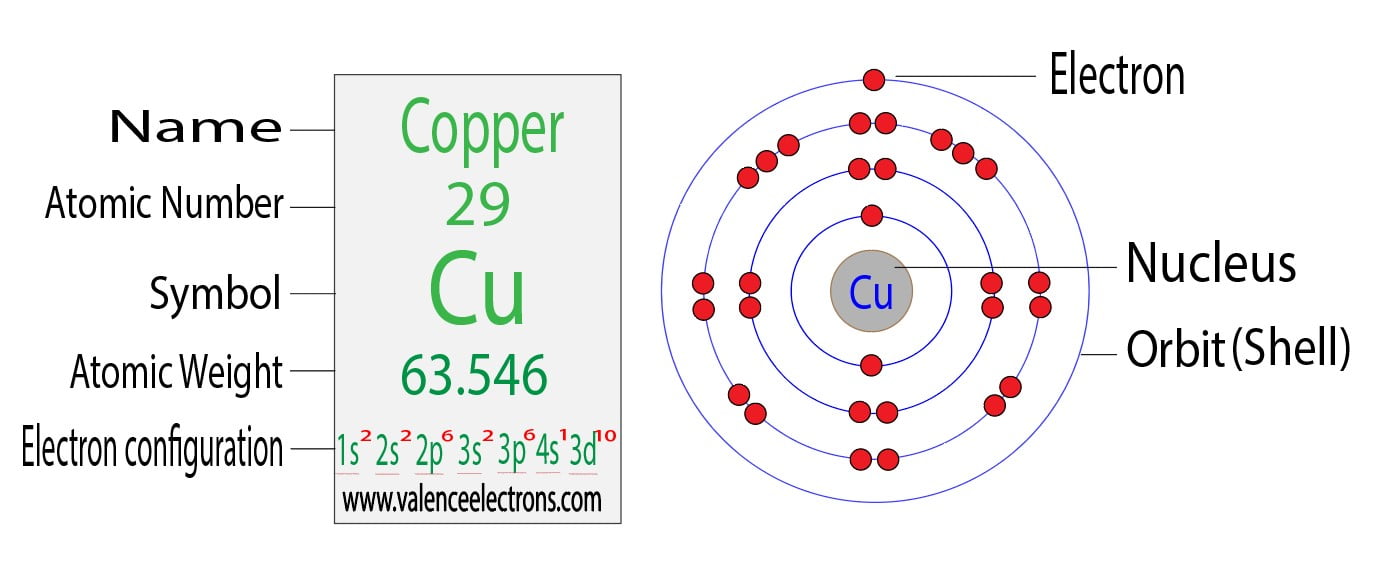
from valenceelectrons.com
Cu = [ar] 4s1 3d10. 312 views 2 years ago 2.3 electronic configuration. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Some of the elements have electron configurations that differ slightly from what our general. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. cu has an anomalous electron configuration. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. Learning outcome 2.3 c) justify the anomalous electronic. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first.
Electron Configuration for Copper (Cu, Cu+, Cu2+)
Copper Electron Configuration Anomaly how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Cr = [ar] 4s1 3d5. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. cu has an anomalous electron configuration. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Cu = [ar] 4s1 3d10. 312 views 2 years ago 2.3 electronic configuration. Learning outcome 2.3 c) justify the anomalous electronic. Some of the elements have electron configurations that differ slightly from what our general. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. the actual electron configurations are: similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Anomaly Cr = [ar] 4s1 3d5. the actual electron configurations are: how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. Learning outcome 2.3 c) justify the anomalous electronic. Cu = [ar] 4s1. Copper Electron Configuration Anomaly.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electron Configuration Anomaly Cu = [ar] 4s1 3d10. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 312 views 2 years ago 2.3 electronic configuration. the libretexts libraries are. Copper Electron Configuration Anomaly.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Anomaly there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. Cu =. Copper Electron Configuration Anomaly.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Anomaly Learning outcome 2.3 c) justify the anomalous electronic. Some of the elements have electron configurations that differ slightly from what our general. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and. Copper Electron Configuration Anomaly.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Anomaly Learning outcome 2.3 c) justify the anomalous electronic. the actual electron configurations are: cu has an anomalous electron configuration. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. Cu = [ar] 4s1 3d10. Some of the elements have electron configurations that differ slightly from what our general. similarly, the observed electron configuration. Copper Electron Configuration Anomaly.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Electron Configuration Anomaly how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Cu = [ar] 4s1 3d10. the actual electron configurations are: Cr = [ar] 4s1 3d5. cu has an anomalous electron configuration. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead. Copper Electron Configuration Anomaly.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Anomaly the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. Cr =. Copper Electron Configuration Anomaly.
From www.slideserve.com
PPT Electron Configurations & The Periodic Table PowerPoint Copper Electron Configuration Anomaly how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Some of the elements have electron configurations that differ slightly from what our general. Learning outcome 2.3 c) justify the anomalous electronic. Cu = [ar] 4s1 3d10. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it. Copper Electron Configuration Anomaly.
From wiringguidefrosts.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper Electron Configuration Anomaly how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Cu = [ar] 4s1 3d10. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Cr = [ar] 4s1 3d5. Learning outcome 2.3 c) justify the anomalous electronic. . Copper Electron Configuration Anomaly.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Anomaly cu has an anomalous electron configuration. the actual electron configurations are: 312 views 2 years ago 2.3 electronic configuration. Cr = [ar] 4s1 3d5. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Learning outcome 2.3 c) justify the anomalous electronic. there is. Copper Electron Configuration Anomaly.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Electron Configuration Anomaly Some of the elements have electron configurations that differ slightly from what our general. cu has an anomalous electron configuration. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Learning outcome 2.3 c) justify the anomalous electronic. there is an apparent anomaly in the electron configuration for copper cu. Copper Electron Configuration Anomaly.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Anomaly the actual electron configurations are: there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. 312 views 2 years ago 2.3 electronic configuration. Cr. Copper Electron Configuration Anomaly.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Electron Configuration Anomaly the libretexts libraries are powered by nice cxone expert and are supported by the department of education. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. Learning outcome 2.3 c) justify the anomalous electronic. how to write the electron configuration for. Copper Electron Configuration Anomaly.
From slideplayer.com
Electronic Structure of Atoms ppt download Copper Electron Configuration Anomaly Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the libretexts libraries are powered by nice cxone expert and are supported by the department of education. Learning outcome 2.3 c) justify. Copper Electron Configuration Anomaly.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Copper Electron Configuration Anomaly Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Cr = [ar] 4s1 3d5. cu has an anomalous electron configuration. Some of the elements have electron configurations that differ slightly from. Copper Electron Configuration Anomaly.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Anomaly Learning outcome 2.3 c) justify the anomalous electronic. Cr = [ar] 4s1 3d5. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. cu has an anomalous electron configuration. the actual electron configurations are: Some. Copper Electron Configuration Anomaly.
From schematicdiagramtelium.z13.web.core.windows.net
Orbital Diagram For Copper Copper Electron Configuration Anomaly Some of the elements have electron configurations that differ slightly from what our general. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. there is an apparent anomaly in the electron configuration for copper cu is [ar]3d 10 4s 1 and not [ar]3d 9 4s 2 because a. . Copper Electron Configuration Anomaly.
From www.youtube.com
ANOMALOUS ELECTRONIC CONFIGURATION OF CHROMIUM AND COPPER YouTube Copper Electron Configuration Anomaly Cu = 1sx2 2sx2 2px6 3sx2 3px6 4sx1 3dx10, it does not follow the. Cu = [ar] 4s1 3d10. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. cu has an anomalous electron configuration. the actual electron configurations are: there is an apparent anomaly in the electron. Copper Electron Configuration Anomaly.